
Highly regulated industries must comply with strict quality and safety standards to prevent defective products, contamination, and other health risks. Because equipment performance directly impacts product quality, maintenance plays a key role in ensuring safe operations and regulatory compliance.
Organizations often rely on computerized maintenance management system (CMMS) software as part of their larger quality management system (QMS) to document, control, and validate maintenance activities that support quality and safety. In this article, we provide an overview of CMMS software validation, explaining what it is and how your organization can use it to maintain regulatory compliance.
What is CMMS Software Validation?

CMMS software validation is a process done to determine if maintenance software complies with the requirements set by the organization, performs its intended functions, and meets the organization’s needs and goals. The purpose of this process is to document that the CMMS meets specifications, has been installed correctly, and can accurately and consistently produce intended results.
In addition, CMMS software validation makes clear how you intend to use the system and identifies potential issues that impact other business processes.
Is CMMS Software Validated?
In our experience, the concept of CMMS software validation causes confusion with first-time buyers. Many ask whether a CMMS is already validated, sometimes thinking that purchasing the system automatically ensures compliance. However, CMMS software cannot be pre-validated, and the software by itself is not compliant with any regulations.
Vendors cannot know exactly how your organization intends to use the system or which specific regulations apply to your operations. Each organization has unique processes, workflows, and compliance requirements, making it impossible for a vendor to pre-validate the software for every scenario.
Who is Responsible for CMMS Software Validation?
Because validation depends on your unique operating environment, your organization is responsible for validating the CMMS. The goal is to ensure it functions as intended, supports compliance needs, and fits into your quality management system (QMS).
While this responsibility lies with you, many vendors provide validation toolkits or support to help guide the process. Organizations often assign a validation team comprised of IT personnel and CMMS power users to manage the CMMS validation process.
The IT department ensures the technical infrastructure is in place to support the CMMS, including servers, networking hardware, computers, devices, and applicable software such as supported operating systems and web browsers. IT also documents technical problems encountered during validation testing.
CMMS power users play the role of project managers for the validation process. They define the scope of validation, identify critical operations, create and execute tests, and document any issues. When problems occur, power users determine the cause and take corrective action. They may also assign other users to perform tests and report results.
Some organizations hire third-party companies to perform software validation tests for them, and CMMS vendors may also offer assistance or services. The cost and effort depend on the number of functions being tested – the fewer the operations, the more affordable the validation. Even when external resources are used, internal IT staff and power users are still valuable for determining technical requirements, managing the project scope, and ensuring the process meets organizational needs.
Learn more about the role of power users in CMMS implementation
How Do You Validate CMMS Software?
While regulatory agencies, like the United States Food and Drug Administration (FDA), require software to be validated, they do not specifically tell organizations how to perform validation. This is because they cannot predict how your organization intends to use the CMMS, so you must show them via the software validation plan and testing. CMMS software validation boils down to:
- Documenting that the CMMS meets specifications
- Installing the CMMS correctly
- Ensuring the CMMS meets your organization’s needs, fulfills its intended use, and functions properly
Disclaimer: Always refer to the documentation provided by the respective compliance agency for guidance.
Though validation may seem daunting, the FDA recommends taking the least burdensome approach, which is defined as “the minimum amount of information necessary to adequately address a relevant regulatory question or issue through the most efficient manner at the right time.”
So, how does one achieve the least burdensome approach? Many organizations follow a basic validation process made up of three sequential stages: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Installation Qualification (IQ)
Installation qualification (IQ) verifies that the CMMS is successfully installed in the environment in which it is meant to be used and documents the supporting hardware, software, and installation procedure. While CMMS vendors provide customers with system requirements, it is up to your organization to obtain the hardware and software required to support the CMMS.
Operational Qualification (OQ)
Operational qualification (OQ) is a documented testing process that verifies that the system does what it is supposed to do. OQ procedures outline the specific actions a user must take in the software to perform an action, the expected outcome, and the actual outcome.
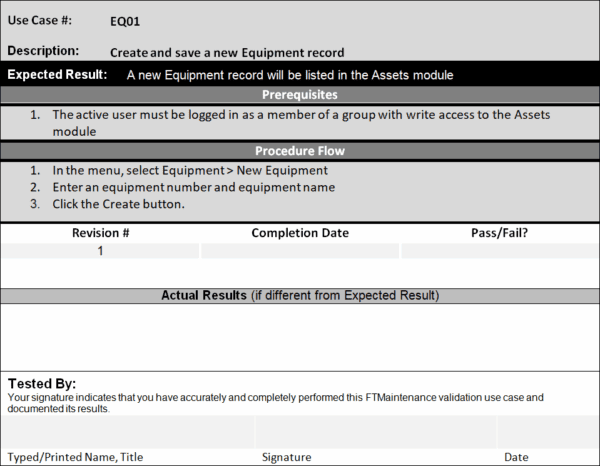
If the actual outcome matches the expected outcome, the test is valid. Differences between the expected and actual results must be documented and rectified. Doing so may involve taking different steps to complete the action (which should be documented) or asking the vendor to resolve the issue.
While it may seem burdensome to perform OQ tests, keep in mind that you only need to test the features and functions that you will use.
Performance Qualification (PQ)
Performance qualification (PQ) determines whether the system performs as intended in real-world conditions. You can think of the previous phase being conducted in a “laboratory setting,” where a limited number of users are testing the software with a small set of data to verify operations. This testing environment is not reflective of real life.
PQ ensures the system is equipped to handle the live load, data volume, bandwidth, storage capacity, and speed required during peak use within a work shift. Ideally, the software responds promptly without freezing or crashing.
What to Do If CMMS Software Fails Validation Testing
Any failures must go through a correction process. The validation team can help determine if the cause of the failure originated from a user, poor test design, incorrect configuration, or even issues with the software itself (such as defects).
User or process issues can be corrected via training, OQ test rewrites, or vendor technical support. If defect fixes are required by the vendor, the organization must retest the software once an update has been delivered. Any changes to software policies, operating procedures, and training documentation need to be updated as well.
Revalidating Software
CMMS software validation is not a one-and-done event. Revalidation is required whenever changes occur that could impact the system’s performance or its role in supporting quality and compliance. Common reasons for revalidation include:
- Upgrading the CMMS to a major new version that changes workflows or core functionality
- Switching to a new CMMS system entirely
- Making major configuration changes that affect validated processes
- Changing IT infrastructure that affects CMMS functionality (e.g., servers, operating systems, hardware)
- Changing how the CMMS is used within your quality management system (QMS) processes
When these situations occur, your organization should review vendor release notes to see what has changed, determine the impact of software changes on current processes, and update validation documentation as appropriate. Only affected operations need revalidation – not the entire software package.
Revalidation is not typically required when there are minor defect fixes that do not affect validated functionality, routine performance or security updates that don’t change workflows, or changes to the user interface (UI) that don’t impact regulated processes.
What Industries Require CMMS Software Validation?

In the United States, software validation is required by organizations that are regulated by the FDA. These industries include:
- Food and beverage
- Pharmaceuticals
- Botanicals
- Medical devices and surgical instruments
- Dental, ophthalmic, and orthopedic equipment and supplies
- Diagnostic substances
- Parts or ingredients used to produce the goods listed above
Maintenance organizations typically focus on the FDA Code of Federal Regulations Title 21, Parts 11 and 820. FDA CFR 21, Part 11 sets rules related to electronic signatures, which a CMMS may use for features like work order approvals. Part 820 describes the requirements for quality management systems, of which a CMMS is part. Organizations that seek voluntary certification, such as ISO 9001 certification, may also be required to validate their CMMS to support quality improvement practices.
Learn more about electronic signature compliance.
Unregulated organizations may also find value in the CMMS validation process in that it can help establish standard operating procedures, build good CMMS practices, and inform software use policies that contribute to higher quality products and processes.
Stay in Compliance with FTMaintenance Select
It often feels like new rules and regulations appear every day, especially in highly-regulated industries. Though CMMS software validation may seem burdensome, the process can be simplified and broken down into smaller pieces. Ultimately, compliance is about how an organization uses their CMMS, not about the software itself. FTMaintenance Select provides an easy-to-use maintenance management platform for documenting, tracking, and managing maintenance activities. Request a demo today.